The Effects of Nuclear Weapons
source link: https://atomicarchive.com/resources/documents/effects/glasstone-dolan/chapter1.html
Go to the source link to view the article. You can view the picture content, updated content and better typesetting reading experience. If the link is broken, please click the button below to view the snapshot at that time.
CHARACTERISTICS OF NUCLEAR EXPLOSIONS
INTRODUCTION
1.01 An explosion, in general, results from the very rapid release of a large amount of energy within a limited space. This is true for a conventional “high explosive,” such as TNT, as well as for a nuclear (or atomic) explosion,1 although the energy is produced in quite different ways (§ 1.11). The sudden liberation of energy causes a considerable increase of temperature and pressure, so that all the materials present are converted into hot, compressed gases. Since these gases are at very high temperatures and pressures, they expand rapidly and thus initiate a pressure wave, called a “shock wave,” in the surrounding medium-air, water, or earth. The characteristic of a shock wave is that there is (ideally) a sudden increase of pressure at the front, with a gradual decrease behind it, as shown in Fig. 1.01. A shock wave in air is generally referred to as a “blast wave” because it resembles and is accompanied by a very strong wind. In water or in the ground, however, the term “shock” is used, because the effect is like that of a sudden impact.
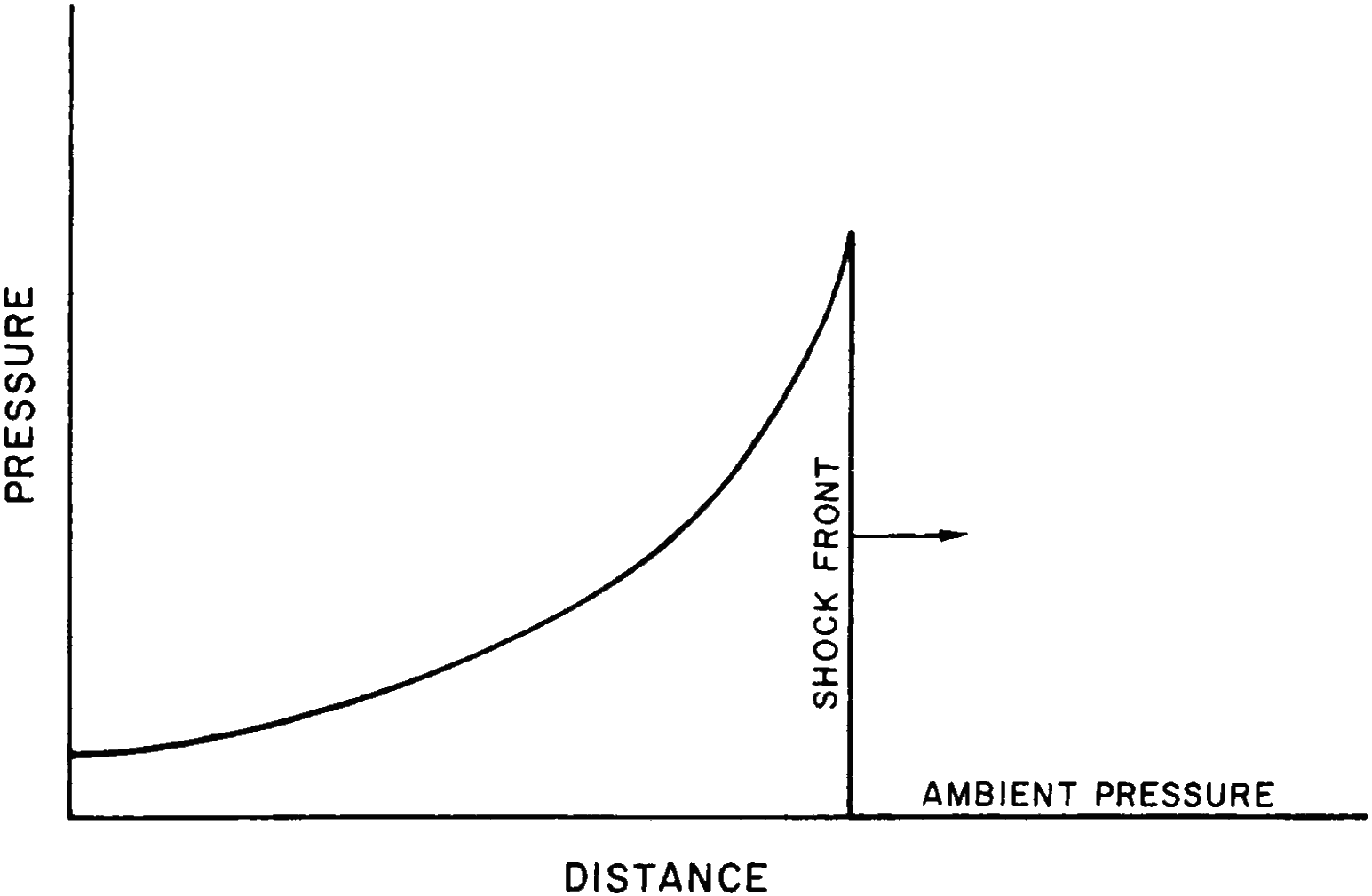
Figure 1.01. Variation of pressure (in excess of ambient) with distance in an ideal shock wave.
1.02 Nuclear weapons are similar to those of more conventional types insofar as their destructive action is due mainly to blast or shock. On the other hand, there are several basic differences between nuclear and high-explosive weapons. In the first place, nuclear explosions can be many thousands (or millions) of times more powerful than the largest conventional detonations. Second, for the release of a given amount of energy, the mass of a nuclear explosive would be much less than that of a conventional high explosive. Consequently, in the former case, there is a much smaller amount of material available in the weapon itself that is converted into the hot, compressed gases mentioned above. This results in somewhat different mechanisms for the initiation of the blast wave. Third, the temperatures reached in a nuclear explosion are very much higher than in a conventional explosion, and a fairly large proportion of the energy in a nuclear explosion is emitted in the form of light and heat, generally referred to as “thermal radiation.” This is capable of causing skin burns and of starting fires at considerable distances. Fourth, the nuclear explosion is accompanied by highly-penetrating and harmful invisible rays, called the “initial nuclear radiation.” Finally the substances remaining after a nuclear explosion are radioactive, emitting similar radiations over an extended period of time. This is known as the “residual nuclear radiation” or “residual radioactivity” (Fig. 1.02).
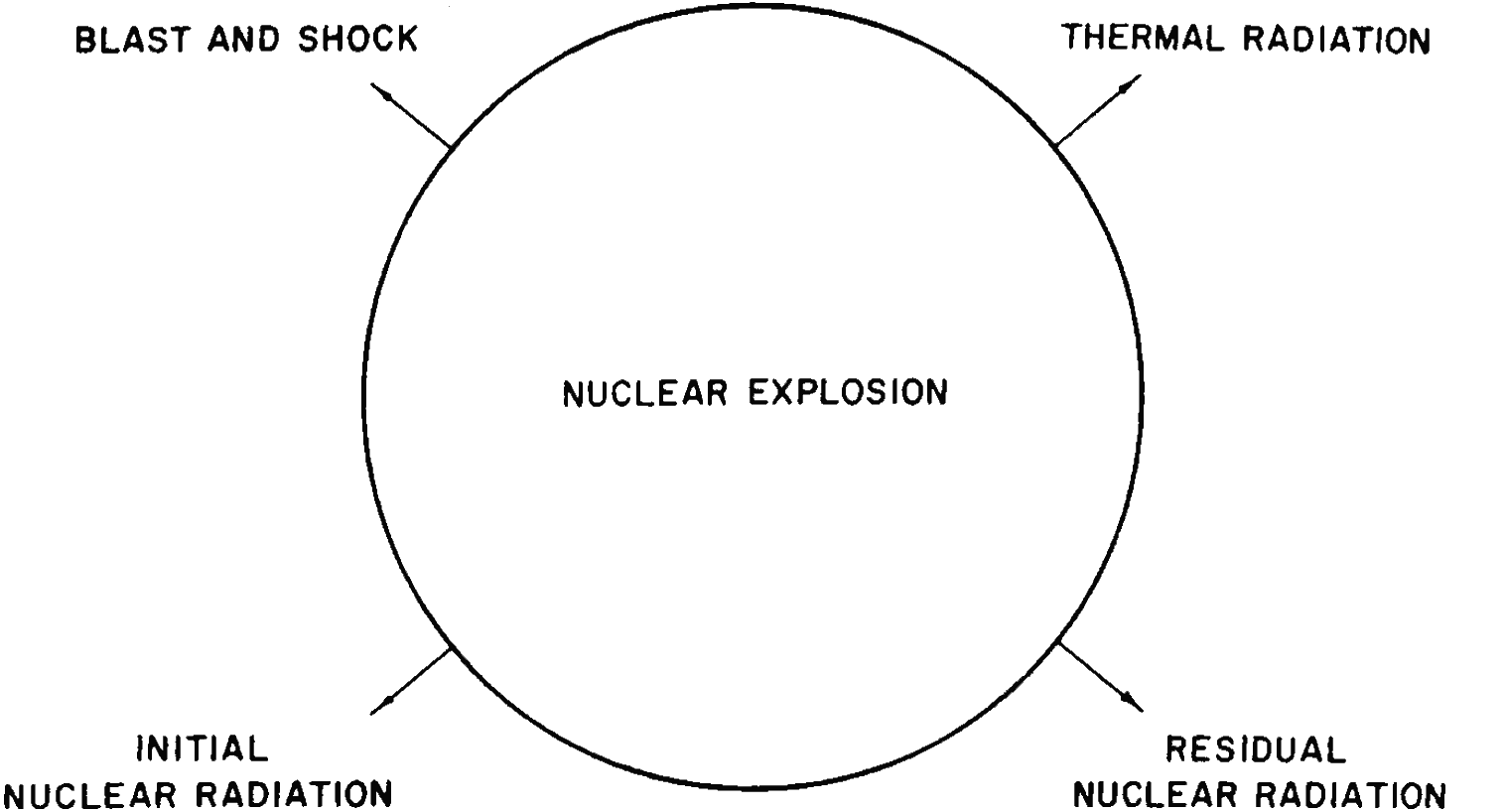
Figure 1.02. Effects of a nuclear explosion.
1.03 It is because of these fundamental differences between a nuclear and a conventional explosion, including the tremendously greater power of the former, that the effects of nuclear weapons require special consideration. In this connection, a knowledge and understanding of the mechanical and the various radiation phenomena associated with a nuclear explosion are of vital importance.
1.04 The purpose of this book is to describe the different forms in which the energy of a nuclear explosion are released, to explain how they are propagated, and to show how they may affect people (and other living organisms) and materials. Where numerical values are given for specific observed effects, it should be kept in mind that there are inevitable uncertainties associated with the data, for at least two reasons. In the first place, there are inherent difficulties in making exact measurements of weapons effects. The results are often dependent on circumstances which are difficult, if not impossible, to control, even in a test and certainly cannot be predicted in the event of an attack. Furthermore, two weapons producing the same amount of explosive energy may have different quantitative effects because of differences in composition and design.
1.05 It is hoped, nevertheless, that the information contained in this volume, which is the best available, may be of assistance to those responsible for defense planning and in making preparations to deal with the emergencies that may arise from nuclear warfare. In addition, architects and engineers may be able to utilize the data in the design of structures having increased resistance to damage by blast, shock, and fire, and which provide shielding against nuclear radiations.
ATOMIC STRUCTURE AND ISOTOPES
1.06 All substances are made up from one or more of about 90 different kinds of simple materials known as “elements.” Among the common elements are the gases hydrogen, oxygen, and nitrogen; the solid nonmetals carbon, sulfur, and phosphorus; and various metals, such as iron, copper, and zinc. A less familiar element, which has attained prominence in recent years because of its use as a source of nuclear energy, is uranium, normally a solid metal.
1.07 The smallest part of any element that can exist, while still retaining the characteristics of the element, is called an “atom” of that element. Thus, there are atoms of hydrogen, of iron, of uranium, and so on, for all the elements. The hydrogen atom is the lightest of all atoms, whereas the atoms of uranium are the heaviest of those found on earth. Heavier atoms, such as those of plutonium, also important for the release of nuclear energy, have been made artificially (§ 1.14). Frequently, two or more atoms of the same or of different elements join together to form a “molecule.”
1.08 Every atom consists of a relatively heavy central region or “nucleus,” surrounded by a number of very light particles known as “electrons.” Further, the atomic nucleus is itself made up of a definite number of fundamental particles, referred to as “protons” and “neutrons.” These two particles have almost the same mass, but they differ in the respect that the proton carries a unit charge of positive electricity whereas the neutron, as its name implies, is uncharged electrically, i.e., it is neutral. Because of the protons present in the nucleus, the latter has a positive electrical charge, but in the normal atom this is exactly balanced by the negative charge carried by the electrons surrounding the nucleus.
1.09 The essential difference between atoms of different elements lies in the number of protons (or positive charges) in the nucleus; this is called the “atomic number” of the element. Hydrogen atoms, for example, contain only one proton, helium atoms have two protons, uranium atoms have 92 protons, and plutonium atoms 94 protons. Although all the nuclei of a given element contain the same number of protons, they may have different numbers of neutrons. The resulting atomic species, which have identical atomic numbers but which differ in their masses, are called “isotopes” of the particular element. All but about 20 of the elements occur in nature in two or more isotopic forms, and many other isotopes, which are unstable, i.e., radioactive, have been obtained in various ways.
1.10 Each isotope of a given element is identified by its “mass number,” which is the sum of the numbers of protons and neutrons in the nucleus. For example, the element uranium, as found in nature, consists mainly of two isotopes with mass numbers of 235 and 238; they are consequently referred to as uranium-235 and uranium-238, respectively. The nuclei of both isotopes contain 92 protons-as do the nuclei of all uranium isotopes-but the former have in addition 143 neutrons and the latter 146 neutrons. The general term “nuclide” is used to describe any atomic species distinguished by the composition of its nucleus, i.e., by the number of protons and the number of neutrons. Isotopes of a given element are nuclides having the same number of protons but different numbers of neutrons in their nuclei.
1.11 In a conventional explosion, the energy released arises from chemical reactions; these involve a rearrangement among the atoms, e.g., of hydrogen, carbon, oxygen, and nitrogen, present in the chemical high-explosive material. In a nuclear explosion, on the other hand, the energy is produced as a result of the formation of different atomic nuclei by the redistribution of the protons and neutrons within the interacting nuclei. What is sometimes referred to as atomic energy is thus actually nuclear energy, since it results from particular nuclear interactions. It is for the same reason, too, that atomic weapons are preferably called “nuclear weapons.” The forces between the protons and neutrons within atomic nuclei are tremendously greater than those between the atoms; consequently, nuclear energy is of a much higher order of magnitude than conventional (or chemical) energy when equal masses are considered.
1.12 Many nuclear processes are known, but not all are accompanied by the release of energy. There is a definite equivalence between mass and energy, and when a decrease of mass occurs in a nuclear reaction there is an accompanying release of a certain amount of energy related to the decrease in mass. These mass changes are really a reflection of the difference in the internal forces in the various nuclei. It is a basic law of nature that the conversion of any system in which the constituents are held together by weaker forces into one in which the forces are stronger must be accompanied by the release of energy, and a corresponding decrease in mass.
1.13 In addition to the necessity for the nuclear process to be one in which there is a net decrease in mass, the release of nuclear energy in amounts sufficient to cause an explosion requires that the reaction should be able to reproduce itself once it has been started. Two kinds of nuclear interactions can satisfy the conditions for the production of large amounts of energy in a short time. They are known as “fission” (splitting) and “fusion” (joining together). The former process takes place with some of the heaviest (high atomic number) nuclei; whereas the latter, at the other extreme, involves some of the lightest (low atomic number) nuclei.
1.14 The materials used to produce nuclear explosions by fission are certain isotopes of the elements uranium and plutonium. As noted above, uranium in nature consists mainly of two isotopes, namely, uranium-235 (about 0. 7 percent), and uranium-238 (about 99.3 percent). The less abundant of these isotopes, i.e., uranium-235, is the readily fissionable species that is commonly used in nuclear weapons. Another isotope, uranium-233, does not occur naturally, but it is also readily fissionable and it can be made artificially starting with thorium-232. Since only insignificant amounts of the element plutonium are found in nature, the fissionable isotope used in nuclear weapons, plutonium-239, is made artificially from uranium-238.
1.15 When a free (or unattached) neutron enters the nucleus of a fissionable atom, it can cause the nucleus to split into two smaller parts. This is the fission process, which is accompanied by the release of a large amount of energy. The smaller (or lighter) nuclei which result are called the “fission products.” The complete fission of I pound of uranium or plutonium releases as much explosive energy as does the explosion of about 8,000 (short) tons of TNT.
1.16 In nuclear fusion, a pair of light nuclei unite (or fuse) together to form a nucleus of a heavier atom. An example is the fusion of the hydrogen isotope known as deuterium or “heavy hydrogen.” Under suitable conditions, two deuterium nuclei may combine to form the nucleus of a heavier element, helium, with the release of energy. Other fusion reactions are described in § 1.69.
1.17 Nuclear fusion reactions can be brought about by means of very high temperatures, and they are thus referred to as “thermonuclear processes.” The actual quantity of energy liberated, for a given mass of material, depends on the particular isotope (or isotopes) involved in the nuclear fusion reaction. As an example, the fusion of all the nuclei present in 1 pound of the hydrogen isotope deuterium would release roughly the same amount of energy as the explosion of 26,000 tons of TNT.
1.18 In certain fusion processes, between nuclei of the hydrogen isotopes, neutrons of high energy are liberated (see § 1.72). These can cause fission in the most abundant isotope (uranium-238) in ordinary uranium as well as in uranium-235 and plutonium- 239. Consequently, association of the appropriate fusion reactions with natural uranium can result in an extensive utilization of the latter for the release of energy. A device in which fission and fusion (thermonuclear) reactions are combined can therefore produce an explosion of great power. Such weapons might typically release about equal amounts of explosive energy from fission and from fusion.
1.19 A distinction has sometimes been made between atomic weapons, in which the energy arises from fission, on the one hand, and hydrogen (or thermonuclear) weapons, involving fusion, on the other hand. In each case, however, the explosive energy results from nuclear reactions, so that they are both correctly described as nuclear weapons. In this book, therefore, the general terms “nuclear bomb” and “nuclear weapon” will be used, irrespective of the type of nuclear reaction producing the energy of the explosion.
ENERGY YIELD OF A NUCLEAR EXPLOSION
1.20 The “yield” of a nuclear weapon is a measure of the amount of explosive energy it can produce. It is the usual practice to state the yield in terms of the quantity of TNT that would generate the same amount of energy when it explodes. Thus, a 1-kiloton nuclear weapon is one which produces the same amount of energy in an explosion as does 1 kiloton (or 1,000 tons) of TNT. Similarly, a 1-megaton weapon would have the energy equivalent of 1 million tons (or 1,000 kilotons) of TNT. The earliest nuclear bombs, such as were dropped over Japan in 1945 and used in the tests at Bikini in 1946, released very roughly the same quantity of energy as 20,000 tons (or 20 kilotons) of TNT (see, however, § 2.24). Since that time, much more powerful weapons, with energy yields in the megaton range, have been developed.
1.21 From the statement in § 1.15 that the fission of 1 pound of uranium or plutonium will release the same amount of explosive energy as about 8,000 tons of TNT, it is evident that in a 20-kiloton nuclear weapon 2.5 pounds of material undergo fission. However, the actual weight of uranium or plutonium in such a weapon is greater than this amount. In other words, in a fission weapon, only part of the nuclear material suffers fission. The efficiency is thus said to be less than 100 percent. The material that has not undergone fission remains in the weapon residues after the explosion.
DISTRIBUTION OF ENERGY IN NUCLEAR EXPLOSIONS
1.22 It has been mentioned that one important difference between nuclear and conventional (or chemical) explosions is the appearance of an appreciable proportion of the energy as thermal radiation in the former case. The basic reason for this difference is that, weight for weight, the energy produced by a nuclear explosive is millions of times as great as that produced by a chemical explosive. Consequently, the temperatures reached in the former case are very much higher than in the latter, namely, tens of millions of degrees in a nuclear explosion compared with a few thousands in a conventional explosion. As a result of this great difference in temperature, the distribution of the explosion energy is quite different in the two cases.
1.23 Broadly speaking, the energy may be divided into three categories: kinetic (or external) energy, i.e., energy of motion of electrons, atoms, and molecules as a whole; internal energy of these particles; and thermal radiation energy. The proportion of thermal radiation energy increases rapidly with increasing temperature. At the moderate temperatures attained in a chemical explosion, the amount of thermal radiation is comparatively small, and so essentially all the energy released at the time of the explosion appears as kinetic and internal energy. This is almost entirely converted into blast and shock, in the manner described in § 1.01. Because of the very much higher temperatures in a nuclear explosion, however, a considerable proportion of the energy is released as thermal radiation. The manner in which this takes place is described later (§ 1.77 et seq.).
1.24 The fraction of the explosion energy received at a distance from the burst point in each of the forms depicted in Fig. 1.02 depends on the nature and yield of the weapon and particularly on the environment of the explosion. For a nuclear detonation in the atmosphere below an altitude of about 100,000 feet, from 35 to 45 percent of the explosion energy is received as thermal energy in the visible and infrared portions of the spectrum (see Fig. 1.74). In addition, below an altitude of about 40,000 feet, about 50 percent of the explosive energy is used in the production of air shock. At somewhat higher altitudes, where there is less air with which the energy of the exploding nuclear weapon can interact, the proportion of energy converted into shock is decreased whereas that emitted as thermal radiation is correspondingly increased (§ 1.36).
1.25 The exact distribution of energy between air shock and thermal radiation is related in a complex manner to the explosive energy yield, the burst altitude, and, to some extent, to the weapon design, as will be seen in this and later chapters. However, an approximate rule of thumb for a fission weapon exploded in the air at an altitude of less than about 40,000 feet is that 35 percent of the explosion energy is in the form of thermal radiation and 50 percent produces air shock. Thus, for a burst at moderately low altitudes, the air shock energy from a fission weapon will be about half of that from a conventional high explosive with the same total energy release; in the latter, essentially all of the explosive energy is in the form of air blast. This means that if a 20-kiloton fission weapon, for example, is exploded in the air below 40,000 feet or so, the energy used in the production of blast would be roughly equivalent to that from 10 kilotons of TNT.
1.26 Regardless of the height of burst, approximately 85 percent of the explosive energy of a nuclear fission weapon produces air blast (and shock), thermal radiation, and heat. The remaining 15 percent of the energy is released as various nuclear radiations. Of this, 5 percent constitutes the initial nuclear radiation, defined as that produced within a minute or so of the explosion (§ 2.42). The final 10 percent of the total fission energy represents that of the residual (or delayed) nuclear radiation which is emitted over a period of time. This is largely due to the radioactivity of the fission products present in the weapon residues (or debris) after the explosion. In a thermonuclear device, in which only about half of the total energy arises from fission (§ 1.18), the residual nuclear radiation carries only 5 percent of the energy released in the explosion. It should be noted that there are no nuclear radiations from a conventional explosion since the nuclei are unaffected in the chemical reactions which take place.
1.27 Because about 10 percent of the total fission energy is released in the form of residual nuclear radiation some time after the detonation, this is not included when the energy yield of a nuclear explosion is stated, e.g., in terms of the TNT equivalent as in § 1.20. Hence, in a pure fission weapon the explosion energy is about 90 percent of the total fission energy, and in a thermonuclear device it is, on the average, about 95 percent of the total energy of the fission and fusion reactions. This common convention will be adhered to in subsequent chapters. For example, when the yield of a nuclear weapon is quoted or used in equations, figures, etc. , it will represent that portion of the energy delivered within a minute or so, and will exclude the contribution of the residual nuclear radiation.
1.28 The initial nuclear radiation consists mainly of “gamma rays,” which are electromagnetic radiations of high energy (see § 1.73) originating in atomic nuclei, and neutrons. These radiations, especially gamma rays, can travel great distances through air and can penetrate considerable thicknesses of material. Although they can neither be seen nor felt by human beings, except at very high intensities which cause a tingling sensation, gamma rays and neutrons can produce harmful effects even at a distance from their source. Consequently, the initial nuclear radiation is an important aspect of nuclear explosions.
1.29 The delayed nuclear radiation arises mainly from the fission products which, in the course of their radioactive decay, emit gamma rays and another type of nuclear radiation called “beta particles.” The latter are electrons, i.e., particles carrying a negative electric charge, moving with high speed; they are formed by a change (neutron -+ proton + electron) within the nuclei of the radioactive atoms. Beta particles, which are also invisible, are much less penetrating than gamma rays, but like the latter they represent a potential hazard.
1.30 The spontaneous emission of beta particles and gamma rays from radioactive substances, i.e., a radioactive nuclide (or radionuclide), such as the fission products, is a gradual process. It takes place over a period of time, at a rate depending upon the nature of the material and upon the amount present. Because of the continuous decay, the quantity of the radionuclide and the rate of emission of radiation decrease steadily. This means that the residual nuclear radiation, due mainly to the fission products, is most intense soon after the explosion but diminishes in the course of time.
TYPES OF NUCLEAR EXPLOSIONS
1.31 The immediate phenomena associated with a nuclear explosion, as well as the effects of shock and blast and of thermal and nuclear radiations, vary with the location of the point of burst in relation to the surface of the earth. For descriptive purposes five types of burst are distinguished, although many variations and intermediate situations can arise in practice. The main types, which will be defined below, are (1) air burst, (2) high-altitude burst, (3) underwater burst, (4) underground burst, and (5) surface burst.
1.32 Provided the nuclear explosion takes place at an altitude where there is still an appreciable atmosphere, e.g., below about 100,000 feet, the weapon residues almost immediately incorporate material from the surrounding medium and form an intensely hot and luminous mass, roughly spherical in shape, called the “fireball.” An “air burst” is defined as one in which the weapon is exploded in the air at an altitude below 100,000 feet, but at such a height that the fireball (at roughly maximum brilliance in its later stages) does not touch the surface of the earth. For example, in the explosion of a 1-megaton weapon the fireball may grow until it is nearly 5,700 feet (1.1 mile) across at maximum brilliance. This means that, in this particular case, the explosion must occur at least 2,850 feet above the earth’s surface if it is to be called an air burst.
1.33 The quantitative aspects of an air burst will be dependent upon its energy yield, but the general phenomena are much the same in all cases. Nearly all of the shock energy that leaves the fireball appears as air blast, although some is generally also transmitted into the ground. The thermal radiation will travel long distances through the air and may be of sufficient intensity to cause moderately severe burns of exposed skin as far away as 12 miles from a 1-megaton explosion, on a fairly clear day. For air bursts of higher energy yields, the corresponding distances will, of course, be greater. The thermal radiation is largely stopped by ordinary opaque materials; hence, buildings and clothing can provide protection.
1.34 The initial nuclear radiation from an air burst will also penetrate a long way in air, although the intensity falls off fairly rapidly at increasing distances from the explosion. The interactions with matter that result in the absorption of energy from gamma rays and from neutrons are quite different, as will be seen in Chapter VIII. Different materials are thus required for the most efficient removal of these radiations; but concrete, especially if it incorporates a heavy element, such as iron or barium, represents a reasonable practical compromise for reducing the intensities of both gamma rays and neutrons. A thickness of about 4 feet of ordinary concrete would probably provide adequate protection from the effects of the initial nuclear radiation for people at a distance of about 1 mile from an air burst of a 1-megaton nuclear weapon. However, at this distance the blast effect would be so great that only specially designed blast-resistant structures would survive.
1.35 In the event of a moderately high (or high) air burst, the fission products remaining after the nuclear explosion will be dispersed in the atmosphere. The residual nuclear radiation arising from these products will be of minor immediate consequence on the ground. On the other hand, if the burst occurs nearer the earth’s surface, the fission products may fuse with particles of earth, part of which will soon fall to the ground at points close to the explosion. This dirt and other debris will be contaminated with radioactive material and will, consequently, represent a possible danger to living things.
1.36 A “high-altitude burst” is defined as one in which the explosion takes place at an altitude in excess of 100,000 feet. Above this level, the air density is so low that the interaction of the weapon energy with the surroundings is markedly different from that at lower altitudes and, moreover, varies with the altitude. The absence of relatively dense air causes the fireball characteristics in a high-altitude explosion to differ from those of an air burst. For example, the fraction of the energy converted into blast and shock is less and decreases with increasing altitude. Two factors affect the thermal energy radiated at high altitude. First, since a shock wave does not form so readily in the less dense air, the fireball is able to radiate thermal energy that would, at lower altitudes, have been used in the production of air blast. Second, the less dense air allows energy from the exploding weapon to travel much farther than at lower altitudes. Some of this energy simply warms the air at a distance from the fireball and it does not contribute to the energy that can be radiated within a short time (§ 1.79). In general, the first of these factors is effective between 100,000 and 140,000 feet, and a larger proportion of the explosion energy is released in the form of thermal radiation than at lower altitudes. For explosions above about 140;000 feet, the second factor becomes the more important, and the fraction of the energy that appears as thermal radiation at the time of the explosion becomes smaller.
1.37 The fraction of the explosion energy emitted from a weapon as nuclear radiations is independent of the height of burst. However, the partition of that energy between gamma rays and neutrons received at a distance will vary since a significant fraction of the gamma rays result from interactions of neutrons with nitrogen atoms in the air at low altitudes. Furthermore, the attenuation of the initial nuclear radiation with increasing distance from the explosion is determined by the total amount of air through which the radiation travels. This means that, for a given explosion energy yield, more initial nuclear radiation will be received at the same slant range on the earth’s surface from a high-altitude detonation than from a moderately high air burst. In both cases the residual radiation from the fission products and other weapon residues will not be significant on the ground (§ 1.35).
1.38 Both the initial and the residual nuclear radiations from high-altitude bursts will interact with the constituents of the atmosphere to expel electrons from the atoms and molecules. Since the electron carries a negative electrical charge, the residual part of the atom (or molecule) is positively charged, i.e., it is a positive ion. This process is referred to as “ionization,” and the separated electrons and positive ions are called “ion pairs.” The existence of large numbers of electrons and ions at high altitudes may have seriously degrading effects on the propagation of radio and radar signals (see Chapter X). The free electrons resulting from gamma-ray ionization of the air in a high-altitude explosion may also interact with the earth’s magnetic field to generate strong electromagnetic fields capable of causing damage to unprotected electrical or electronic equipment located in an extensive area below the burst. The phenomenon known as the “electromagnetic pulse” (or EMP) is described in Chapter XI. The EMP can also be produced in surface and low air bursts, but a much smaller area around the detonation point is affected.
1.39 If a nuclear explosion occurs under such conditions that its center is beneath the ground or under the surface of water, the situation is described as an “underground burst” or an “underwater burst,” respectively. Since some of the effects of these two types of explosions are similar, they will be considered here together as subsurface bursts. In a subsurface burst, most of the shock energy of the explosion appears as underground or underwater shock, but a certain proportion, which is less the greater the depth of the burst, escapes and produces air blast. Much of the thermal radiation and of the initial nuclear radiation will be absorbed within a short distance of the explosion. The energy of the absorbed radiations will merely contribute to the heating of the ground or body of water. Depending upon the depth of the explosion, some of the thermal and nuclear radiations will escape, but the intensities will generally be less than for an air burst. However, the residual nuclear radiation, i.e., the radiation emitted after the first minute, now becomes of considerable significance, since large quantities of earth or water in the vicinity of the explosion will be contaminated with radioactive fission products.
1.40 A “surface burst” is regarded as one which occurs either at or slightly above the actual surface of the land or water. Provided the distance above the surface is not great, the phenomena are essentially the same as for a burst occurring on the surface. As the height of burst increases up to a point where the fireball (at maximum brilliance in its later stages) no longer touches the land or water, there is a transition zone in which the behavior is intermediate between that of a true surface burst and of an air burst. In surface bursts, the air blast and ground (or water) shock are produced in varying proportions depending on the energy of the explosion and the height of burst.
1.41 Although the five types of burst have been considered as being fairly distinct, there is actually no clear line of demarcation between them. It will be apparent that, as the height of the explosion is decreased, a high-altitude burst will become an air burst, and an air burst will become a surface burst. Similarly, a surface burst merges into a subsurface explosion at a shallow depth, when part of the fireball actually breaks through the surface of the land or water. It is nevertheless a matter of convenience, as will be seen in later chapters, to divide nuclear explosions into the five general types defined above.
Recommend
About Joyk
Aggregate valuable and interesting links.
Joyk means Joy of geeK