

同济大学等Nature子刊:酸性电解水,低铱阳极可理性设计
source link: https://www.qianzhan.com/analyst/detail/329/230103-4f6496f2.html
Go to the source link to view the article. You can view the picture content, updated content and better typesetting reading experience. If the link is broken, please click the button below to view the snapshot at that time.
同济大学等Nature子刊:酸性电解水,低铱阳极可理性设计

作者|eChemStore 来源|eChemStore(ID:gh_3d533100456a)
论文简介:
设计活性稳定、经济高效的酸性析氧(OER)电催化剂是研制质子交换膜水电解槽的关键。同济大学 Jiwei Ma、Technical University Berlin的Peter Strasser和Max Planck Institute for Chemical Physics of Solids的Zhiwei Hu在Nature Communications上报道了一种通过机械化学方法制备的铱单原子嵌入的钴氧化物(Ir-CoO)。Operando X射线吸收光谱表明,Ir原子在反应过程中被部分氧化为活性Ir,同时Ir和Co原子及其桥接的亲电O配体作为活性位点,共同提高了性能。理论计算进一步揭示了孤立Ir原子能有效地提高电子电导率和优化能垒。因此,在酸性条件下,Ir-CoO表现出明显高于基准IrO的质量活性和催化翻转频率(TOF)。此外,催化剂制备可以很容易地扩大到克级。本文发展的方法揭示构建单一贵金属原子可能获得具有成本效益且有实际应用的金属氧化物OER催化剂。
图文速览:

Fig. 1: Structural characterizations of Ir-CoO. a SEM, (b) TEM, (c) AFM and (d) HR-TEM images of Ir-CoO. Inset in (c) is the height profile of Ir-CoO along the dashed line. e AC HAADF-STEM image and the corresponding FFT image (inset) of Ir-CoO. f The enlarged area in (e), with the Ir single atoms marked in circles, and (g) intensity profiles along the dashed rectangles. h 3D atom-overlapping Gaussian function fitting mapping of the selected area in (e). i AC HAADF-STEM image and the corresponding elemental mappings of Ir-CoO.
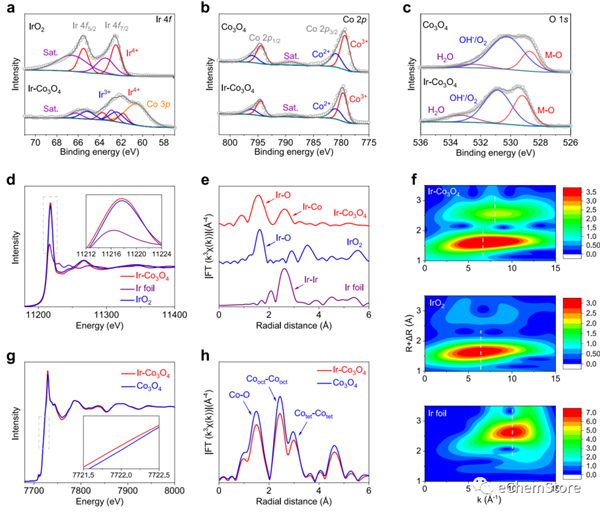
Fig. 2: Electronic and atomic structure analysis of Ir-CoO. a Ir 4f XPS spectra of Ir-CoO and IrO. b Co 2p XPS spectra and (c) O 1 s XPS spectra of Ir-CoO and CoO. d XANES spectra of Ir-L edge on Ir-CoO, Ir foil and IrO. d The normalized Ir-L edge XANES spectra of Ir-CoO, Ir foil and IrO. e Ir-L edge EXAFS spectra and (f) corresponding wavelet-transformed k-weighted EXAFS spectra of Ir-CoO, Ir foil and IrO. g The normalized Co-K edge XANES spectra and (h) Co-K edge XANES spectra of Ir-CoO and CoO.
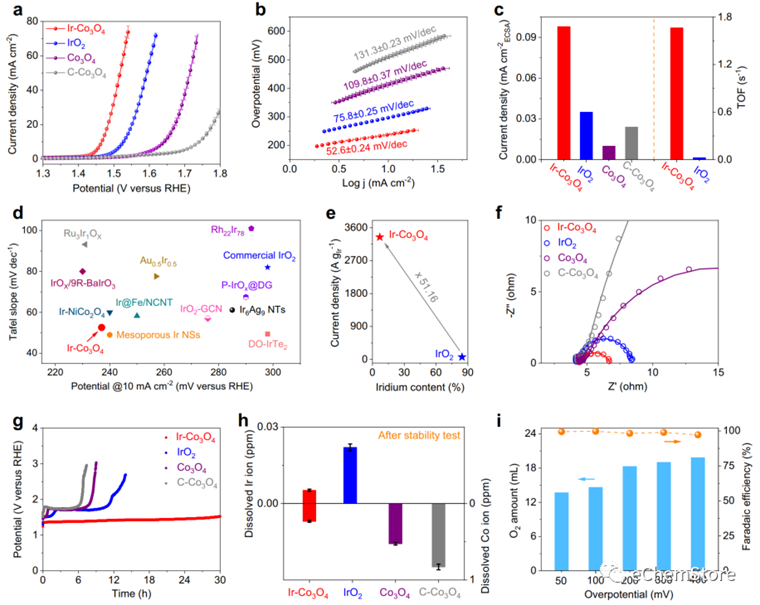
Fig. 3: Electrochemical OER of Ir-CoO. a Polarization curves of Ir-CoO, IrO, CoO and C-CoO in 0.5 M HSO at a scanning rate of 5 mV s. b Tafel plots derived from the polarization curves in (a). c Corresponding specific activities of Ir-CoO, IrO, CoO and C-CoO; TOF values of Ir-CoO and IrO at the overpotential of 300 mV. d Comparison of overpotentials and Tafel plots in reported Ir-based OER electrocatalysts. e Comparison of the mass activities normalized to Ir mass on Ir-CoO and IrO. f EIS Nyquist plots and fitting curves of samples recorded at the 1.43 V vs. RHE. g Chronopotentiometric measurements of Ir-CoO, IrO, CoO and C-CoO at 10 mA cm, carbon paper was used as the catalyst support. h Dissolved Ir (left-y axis) and Co (right-y axis) ion concentrations measured for Ir-CoO, IrO, CoO and C-CoO in the electrolyte by ICP-OES. i Generated O amount and corresponding Faradaic efficiency over a range of overpotentials on Ir-CoO. Note: error bars represent the standard deviation of three independent measurements.
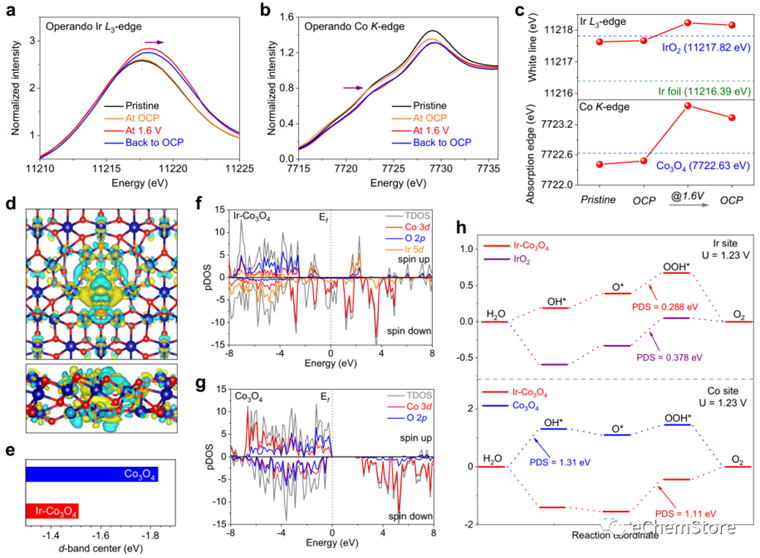
Fig. 4: Operando XANES analysis and energetic properties of Ir-CoO for OER. a The Ir L-edge XANES and (b) the Co K-edge XANES of Ir-CoO measured in 0.5 M HSO under pristine state, OCP, OER operating condition of 1.6 V and OCP after OER operation. Ir L-edge XANES of Ir foil and IrO, and Co K-edge XANES of CoO are used as references. c The white line positions and the absorption edge positions of all measured XANES. d The top and side panels of charge density difference on Ir-CoO. e Comparison of the d-band center of Ir-CoO and CoO. Corresponding Co 3d, O 2p and Ir 5d pDOS spectra of (f) Ir-CoO and (g) CoO. h The Gibbs free energy diagrams of the four-electron OER process on the Ir sites and Co sites of these catalysts under the applied overpotentials of 1.23 V vs. RHE, respectively.
论文总结:
综上所述,作者通过简单经济的机械力化学方法成功合成了活性Ir-CoO OER催化剂。AC HAADF-STEM表征和XAS技术均证实了Ir单原子均匀地分散在CoO基体材料中。结果表明,在酸性介质中掺杂微量Ir原子后,CoO的过电位(412 mV)急剧下降至236 mV,并延长了其长期稳定性。值得注意的是,Ir-CoO的归一化质量活度和TOF均高于商业IrO。通过XANES分析,作者认为,在阳极电压下,Ir单原子被部分激活到更高的价态,同时Co和Ir原子及其亲电O配体作为活性位点在OER过程中协同负责电荷转移。DFT计算进一步证明,高价Ir诱导氧配体的亲电性提高了OER的电子导电性,降低了OER的能垒,提高了OER的活性。此外,与大多数单原子催化剂的传统合成方法相比,Ir-CoO的制备方法可以很容易地扩大到克级生产,活性损失可以忽略不计。这项研究揭示了一种有前景的、具有成本效益和节能的催化剂的合理设计概念,用于促进酸性介质中的OER反应,可能对工业酸性电解水制氢有用。
全文链接:
https://www.nature.com/articles/s41467-022-35426-8
编者按:本文转载自微信公众号:eChemStore(ID:gh_3d533100456a),作者:eChemStore
Recommend
About Joyk
Aggregate valuable and interesting links.
Joyk means Joy of geeK