Ginkgo Bioworks: The Organism Company
source link: https://www.notboring.co/p/ginkgo-bioworks-the-organism-company?s=r
Go to the source link to view the article. You can view the picture content, updated content and better typesetting reading experience. If the link is broken, please click the button below to view the snapshot at that time.
Ginkgo Bioworks: The Organism Company
Elliot Hershberg joins Not Boring and introduces us to synthetic biology
Welcome to the 1,073 newly Not Boring people who have joined us in the last couple weeks! If you haven’t subscribed, join 130,985 smart, curious folks by subscribing here:
🎧 If you’d rather listen to this essay, head over to Spotify or Apple Podcasts
Today’s Not Boring is brought to you by… Rows
You know I’m bullish on all things web2, web3, web[INSERT NUMBER]. You might even say I’m an Optimist. But let's face it: behind every crypto project, blockchain and DAO there is a spreadsheet. There is always a spreadsheet..
Rows is the spreadsheet for the Slack generation. Last year, I wrote that Excel Never Dies. I may have spoken too soon. Rows very much wants Excel to die. It has a shot: it’s one of the most “Holy shit 🤯” new product experiences I’ve had in a long time.
It’s kind of like if Excel, Zapier and a BI tool had a baby, plus access to databases that normally live behind paywalls. And it’s all without code.
This time I teamed up with the Rows team to create a spreadsheet for retail investors: a head-to-head comparison of the financial performance of public companies.
You can play around with it here and copy it for free to your Rows account.
Hi friends 👋,
Happy Monday!
Last week, I wrote a piece on Optimism that seemed to resonate with a lot of people. Beyond the headlines, there are pretty mind-blowing advances happening across the board – from space to web3 to climate to biotech and beyond. Optimism isn’t blind faith that everything will work out; it’s a belief that by experimenting and accumulating knowledge, humans can overcome the inevitable challenges we’ll face.
The best way I know to make the world more optimistic is for Not Boring to tell the stories of, and invest in, the people and companies solving those challenges. A lot of those companies work at the cutting edge of science and engineering, so we’ve expanded the Not Boring team by bringing on two Research Analysts who have the technical chops to help make sense of it all:
Elliot and Rahul will work with us at Not Boring Capital, and even better for all of us, write about companies, technologies, and ideas driving their spaces in Not Boring. These fields have the potential to fundamentally change the way we live, and I want Not Boring to be the place that you learn about them. In addition to being technically strong, they’re both excellent writers. You’ll meet Rahul in a few weeks when he publishes his first piece for Not Boring.
Today, Elliot is going to take us on a journey through the world of synthetic biology by analyzing one of its most innovative companies: Ginkgo Bioworks. Elliot knows what he’s talking about. In addition to writing one of the best biotech newsletters on Substack, The Century of Biology (it’s really good: subscribe now), he’s currently a PhD student in the Department of Genetics at Stanford. It’s rare to find someone so technically strong who’s also so good at communicating what’s happening in his industry and imagining where it might be headed. In today’s piece, you’ll see what I mean.
Synthetic biology might let humans harness “the awesome power of biology to create an abundant future,” as Elliot writes. Or as a Ginkgo site, syntheticbiology.com, asks: “Imagine a future designed with biology. What if you could grow anything?”
Let’s get to it.
Ginkgo Bioworks: The Organism Company
If we were visited by intelligent life from elsewhere in the Universe, what would be the most embarrassing aspect of our current technological capacity? In some ways, it would be hard to pick. Despite the fact that we all carry supercomputers in our pockets, we dig up dead dinosaurs and burn them in combustion engines to power our cars, trains, planes, and cities. To make new molecules—drugs, compounds for agriculture, or any other chemical product—we rely on complex industrial systems that create far more waste than desired. Our approach to construction has escalating costs and can’t scale to support a growing population.
What if we figured out a way to do things differently? What if we found a way to have a more productive relationship with Nature? What if we could learn to grow anything?
This is the vision of synthetic biology. The researchers, engineers, labs, and companies in this discipline are aiming to dramatically change our approach to creating food, materials, chemicals, and physical structures by harnessing the only functioning nanotechnology that we know of: living systems.
Ginkgo Bioworks—The Organism Company—is a leading force in this new field. Their ambitious goal of becoming the first platform company in synthetic biology has generated strong reactions, both positive and negative. In this essay, I want to explore some of the Big Ideas that this company is pursuing, and provide a lens into what could be possible for the broader field of synthetic biology in the future. Today, Ginkgo’s platform is being used to engineer microbes that can be used to make agriculture more sustainable, grow valuable chemicals, and deliver therapeutics in a targeted fashion.
Given the utter insanity of the current markets and the fact that this is a publicly traded company, I want to be absolutely clear: this is not investment advice. My goal is to provide an honest and accurate assessment of the incredibly important experiment that Ginkgo is running for the biotechnology industry.
Here’s a map of where we’re going to go in order to explore this:
What is synthetic biology?
How biotechs makes money
What does The Organism Company do?
Major open questions 🐻
An experiment in cooperative work structure
What will the world look like if Ginkgo succeeds?
Let’s jump in! 🧬🌱
What is synthetic biology?
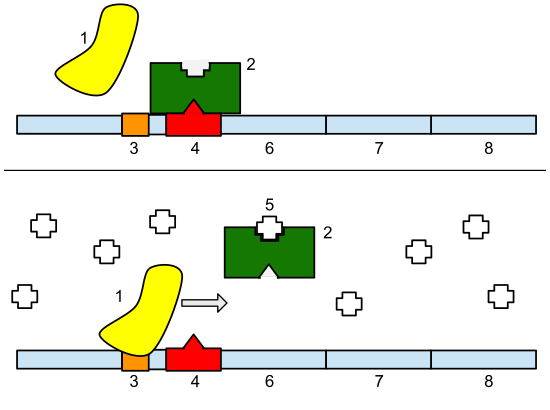
Let’s talk about circuit design. A good circuit does a few things. It should receive information and perform a computation based on the input to produce a reliable output. Input, computation, output. One of my favorite circuits senses an environmental gradient as input, and computes on that information to determine which set of machines it should turn on or off. I’m not describing a digital circuit; this is a genetic circuit found in Nature called the lac operon.
Think back to high school biology. Remember that cells encode instructions in DNA called genes. These instructions are copied into messenger RNA molecules that ultimately serve as a template for creating proteins, which are the machines that do most of the work inside of cells. In bacterial cells, this simple circuit involves a repressor component (shown in green) that is able to sense the sugar gradient in the cell, and compute on that information to control which set of genes gets turned on. The computation is: if lactose is present, the polymerase (yellow) can bind to the promoter sequence (orange) and turn on the genes to metabolize it. Else, keep the genes turned off.
Learning that these types of genetic circuits exist was a pretty big deal, and the French scientists François Jacob and Jacques Monod nabbed the 1965 Nobel Prize in Physiology for figuring it out. Even this early on, Jacob and Monod postulated that in principle it should be possible to create new circuits by combining regulatory DNA systems in different ways.
In the following decades, we developed several important tools for doing this. Molecular cloning unlocked the ability to assemble new sequences of DNA, and PCR let us make copies of DNA. These technologies led to the birth of genetic engineering, and arguably modern biotechnology at large. Genentech—previously trading under the $DNA stock ticker now held by Ginkgo before operating as a subsidiary of Roche—became a blockbuster success by using genetic engineering to create bacterial cells that produced human insulin.
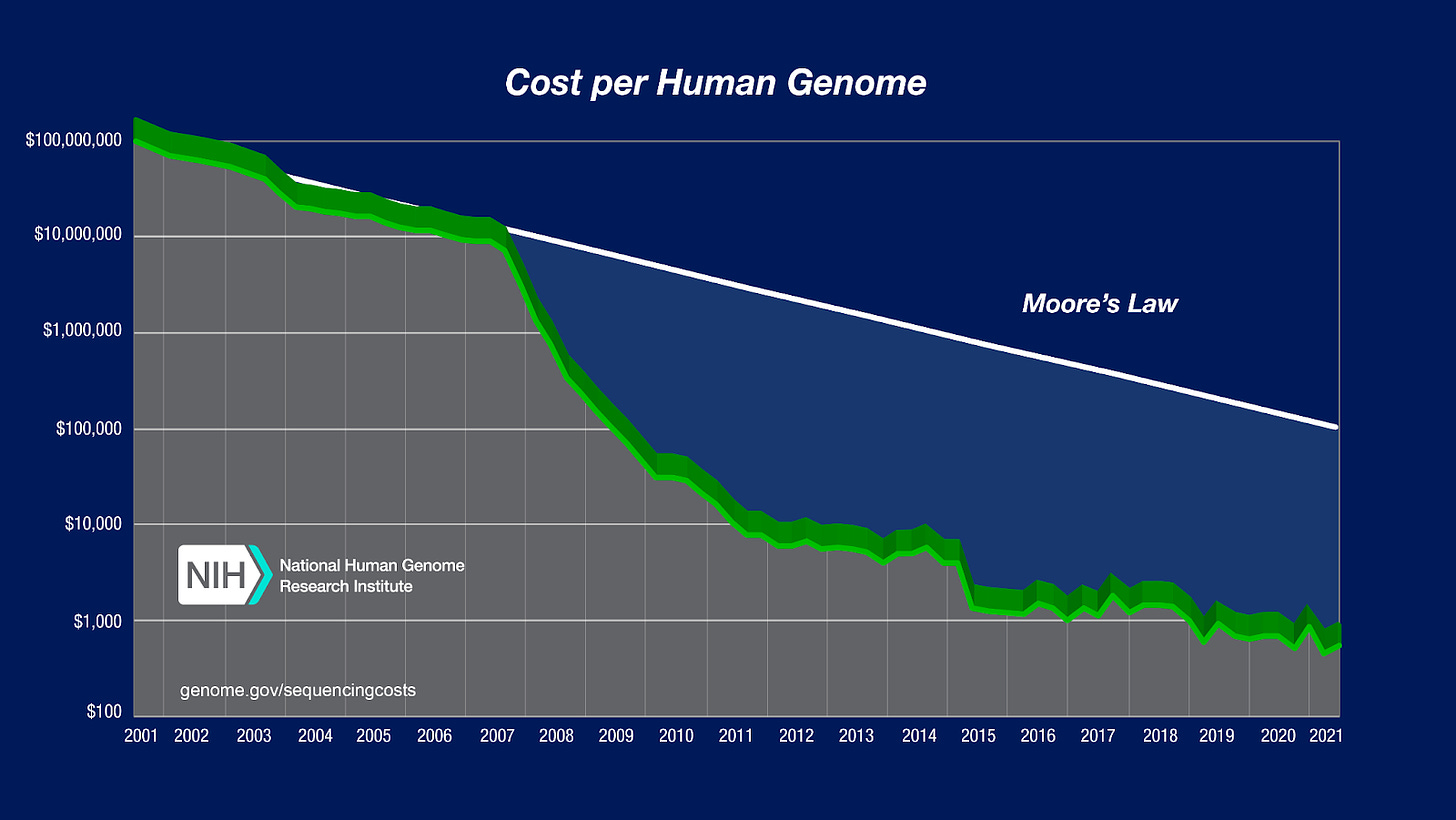
While this was a defining success for biotech as an industry, the synthesis of insulin represented the peak of design complexity that was possible at the time. But as our ability to modify organisms remained limited, our ability to measure organisms underwent a mindblowing revolution. In 1990, the United States Department of Energy and the National Institutes of Health launched a $3 billion dollar project to sequence the entirety of the DNA bases that make up a human—which we call a genome. This marked the start of one of the craziest cost declines in the history of technology.
Many people in tech are familiar with Moore’s Law, which was the prediction that the number of transistors in a dense integrated circuit doubles about every two years. In practice, this means that we have ended up with computers in our pockets that are more powerful than the room-sized computers scientists used to get to the moon. The graph above—which is used in more than two thirds of genomics lectures—shows how DNA sequencing cost declines massively outperformed that rate. While the first human genome took a decade to sequence and cost $3 billion dollars, graduate students now routinely sequence genomes in a day for only $1,000 dollars or less. In the past few weeks, Ultima Genomics came out of stealth and announced their goal to usher in the $100 genome.
This change in measurement technology was a really big deal. After decades studying individual genes, geneticists began studying thousands of genes at a time. We also learned to adapt some of the fundamental tech to make high-throughput measurements of more parts of the cell, like RNA, metabolites, and proteins. This was so transformative that it launched an entire new field called systems biology with the goal of making unifying models describing all of these measurements. As the Stanford bioengineer Markus Covert likes to say, “When I was growing up, biology was the science that you would take if you didn’t want to learn any math. Now that’s not the case anymore.” With a new stack of tools, molecular biology evolved into a quantitative discipline.
Synthetic biology was launched largely in response to this paradigm shift. The goal was to transform genetic engineering into a discipline capable of building new biological circuits from the ground up. The idea was to develop the capacity to engineer new organisms.
The Century of Biology started with a bang when several studies were published in January 2000 describing the successful development of engineered genetic circuits—finally realizing the vision of Jacob and Monod. Over the last decades, synthetic biology has matured as a discipline, and has started to develop reusable community standards, which has decreased the overhead of launching new projects. One of the best demonstrations of this is iGEM—the International Genetically Engineered Machine competition—where undergraduates and high schoolers around the world compete to implement their best ideas in DNA code. Recent winners have developed new ways to manufacture fragrances, materials, and diagnostics, all using the shared iGEM building blocks.
So, what is synthetic biology? It depends on who you ask. According to Christina Agapakis, it is “the practice of engineering life.” For one of the pioneers of the field named Drew Endy, synthetic biology is focused on a meta-goal: to systematically improve our ability to use biology as an engineering substrate.
Regardless of how you define it, the field of synthetic biology is comprised of dreamers and doers. The vision expands as far as you can imagine: designing programmable cell therapies, learning how to grow rocket ships and space stations, and bringing woolly mammoths back from extinction. In practice, there is a relentless focus on discovering new molecular tools, scaling DNA synthesis, and creating systems of re-usable standards and components. With the current rate of progress and the boundless possibilities on the horizon, Eric Schmidt has said that “your industry is at the same stage mine was in 40 years ago when I started my career in tech.”
How biotechs make money
So, synthetic biology is focused on learning how to build things using biology. Before analyzing Ginkgo, it’s also important to think about how the biotech industry makes money. By and large, the revenue that flows through the ecosystem comes from selling drugs.
This seems like a simple statement, but it has non-obvious implications. Drugs are a very unique product. They require an enormous amount of R&D, and have to make it through many expensive hurdles to demonstrate efficacy and safety before being approved. After all of this time and cost there is another problem: the actual chemicals can be manufactured incredibly cheaply, which is why generic versions of drugs can be sold for as low as a dollar a day.
Companies are incentivized to develop new drugs by the promise of patent and market exclusivity. Drug companies need to recoup all of their R&D and regulatory costs in the time window of their exclusivity—and have also found ways to extend the time that the window lasts.
With this incentive structure, biotech has become an industry with public companies that have hundreds of employees and no product. The core value of these companies is the assets in their clinical pipeline—which stand the chance to become blockbuster drugs with market exclusivity if they are approved. Approved assets are primarily sold to major pharmaceutical companies.
From a business strategy perspective, this has meant that most value capture comes from pursuing highly specific vertical solutions—new drugs for specific diseases. Some of the most successful horizontal companies in the industry have focused on selling specific instruments or reagents to research labs and companies. An example of this type of company is Illumina, which sells DNA sequencers and the reagents necessary to generate new data. As biotech has matured, platform biotechs have emerged that are aiming to change this dynamic by offering more expansive R&D infrastructure that would have traditionally been built in-house by individual companies.
We’ve all benefited enormously from platform biotechs. Moderna isn’t a vaccine company, it is an mRNA therapeutics platform company. Before COVID, Moderna had created multiple internal portfolio companies that were pursuing specific vertical applications of their core technology. Because of the flexibility of their RNA tech, they were able to design their COVID vaccine in a matter of hours after receiving the DNA sequence of the virus. With the emergency vaccine approval, we saw one of the fastest transitions from R&D to clinical administration in history.
Some platform companies focus on providing R&D services to biotechs instead of launching their own internal companies like Moderna. Adimab, for example, partners with biotechs and pharma companies to help them make antibody therapies so that they don’t have to do it themselves. As one of the Adimab co-founders Tillman Gerngross put it, “Airlines don’t build their own airplanes, and there’s a good reason for that. They focus on the particular thing they’re good at and leave other parts to others. And I think our industry is maturing in a similar way.” This evolution will be essential for understanding Ginkgo.
What does The Organism Company do?
While biotech was heavily focused on making drugs, synthetic biologists continued to spend their time developing better ways to engineer organisms. Many of the founders of the discipline entered into the world of biology from computer science, electrical engineering, and other fields with a deep emphasis on general solutions and explicit standards.
These early leaders were deeply optimistic about the technological progress being made in both DNA sequencing and synthesis that provided the essential tooling to manipulate and engineer organisms. Engineers like Tom Knight and Drew Endy at MIT talked about what was necessary to take genetic engineering to the next level. In order to operate like other engineering disciplines, the field needed several new concepts.
First, builders needed standardized parts with predictable behavior that they could compose and assemble in new ways. There also needed to be meaningful abstraction layers—analogies were often made to modern programming languages that let programmers focus on logic instead of machine code. We needed to move from worrying about raw bases of DNA—ATTCGGATA—to programs closer to the lac operon. Another core emphasis was to more effectively coordinate labor—separating designers of genetic programs from the builders directly compiling it.
In other words, biology needed to develop the necessary infrastructure to become a true engineering discipline.
The iGEM competition has been one of the most vibrant communities for making progress on these foundational goals. The teams can spend their time focusing on their most ambitious ideas because they are building using a shared Parts Registry, a Distribution Kit, and a set of Assembly Standards. Instead of worrying about the behavior of every single enzyme in a new circuit, they can take enzymes off the shelf and spend their effort brainstorming how best to program molds into assembly lines producing foods, detergents, and medicines. This ethos is reflected in the beautifully nerdy fact that the iGEM grand prize is the BioBrick Trophy: a giant aluminum brick that looks like a Lego block.
Even as synthetic biology and iGEM gained traction, some of the leading professors like Tom Knight still struggled to get their work funded. The work didn’t neatly align with the core priorities of any major grant agencies and was very early. At one point, an MIT grad student named Jason Kelly asked Tom, “What do you think if we started a company?” Tom responded, “What would you think if I joined you?” While most professors were happy to “spin out” companies from their labs, Tom Knight wanted to dive in and join the adventure.
Ginkgo Bioworks was born from this conversation. The company was launched with a rockstar team of MIT scientists with Tom Knight, Jason Kelly, Reshma Shetty, Barry Canton, and Austin Che as co-founders. The only open question was what the company would actually do.
In the early years, Ginkgo searched broadly for how best to develop a profitable business. There wasn’t a sufficient market for them to focus on engineering problems like providing DNA assembly services, so they did what biotechs have done: focus on vertical solutions. Just like in therapeutics, existing companies using biology to do manufacturing focused on developing specific products to generate revenue. These included things like beauty and skincare products, sugar substitutes, and chemicals. One of Ginkgo’s early product successes took inspiration from the iGEM Eau d’e coli project that used E. coli to synthesize fragrances.
As biotech started to accelerate and morph into an engineering discipline, Silicon Valley hackers started to pay attention. In 2014, Y Combinator included a section about biotech for the first time in their Request for Startups saying:
It’s still early, but it seems like we’re finally making real progress hacking biology. There are so many directions this can go—fighting disease, slowing aging, merging humans and computers, downloading memories, genetic programming, etc. We are certain that this is going to be a surprising, powerful and controversial field over the next several decades—it feels a little bit like microcomputers in the 1970s.
Ginkgo finally had an audience for their vision of making biology programmable. That year, they became the first biotech ever funded by Y Combinator. Over the course of their time at YC, they iterated rapidly on their business model and landed on the strategy that they are still driving forward today: “Instead of producing chemicals themselves, they’d engineer organisms and license them to other companies.” Ginkgo is aiming to be the defining platform company for synthetic biology.
This is a crucial point: Ginkgo’s platform is structured to make revenue in three ways: 1) through Cell Programs where Ginkgo provides engineered organisms to customers in exchange for cash and either downstream royalties or equity, 2) Platform Ventures where new companies are launched, or 3) Structured Partnerships where Ginkgo provides R&D on a recurring basis for existing companies.
What foundational components are necessary when building a technological platform for programming cells? You definitely need to be able to write a lot of DNA to create new programs. Ginkgo has developed extensive in-house DNA synthesis capacities, and is the largest customer of the synthetic DNA provider Twist Bioscience. You also need to read a lot of DNA to test how programs work—so automated DNA sequencing and other high-throughput measurements are essential.
Handling the scale of this type of engineering operation introduces totally new challenges. It becomes impossible to rely on the existing approach of having graduate students and staff scientists spend their days transferring small volumes of liquid using handheld pipettes. From the start, the vision was to industrialize genetic engineering using automation and software. In his 2014 YC demo day presentation, Ginkgo CEO Jason Kelly said:
We’ve used robotic engineering and software to reduce the cost of genetic engineering by a factor of 5x in the last two years. This is the beginning of a Moore’s law for genetic engineering…. The last 20 years of biotechnology have been the punch card era of biotech: slow, manual, tedious programming of organisms…. Imagine what can be done with a modern programming stack on top of biology.
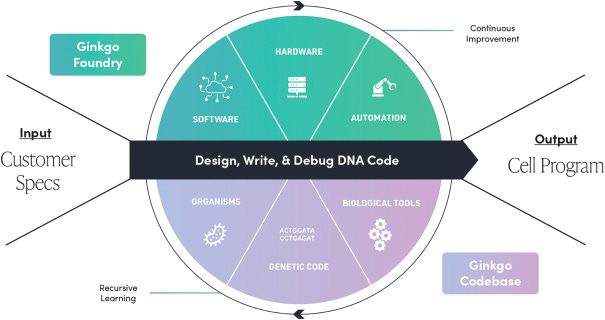
Today, Ginkgo organizes their company structure around the goal of rapidly using and improving this central technology stack. The central focus of the platform is on Cell Programs.
Here, Ginkgo works with customers who have interest in developing a new organism for a specific industrial purpose. The desired outcomes for the program are clarified in an initial set of customer specifications. The specs are handed off to cell designers at Ginkgo, who leverage the Codebase of organisms, DNA sequences, and genetic components—like in iGEM—to engineer a new organism with the desired properties.
The next stage of the process is the handoff between the design team and the build team, which leverages the software, hardware, and automation of Ginkgo’s Foundry. Here, the designs are physically instantiated and validated using their DNA synthesis and sequencing infrastructure, and the designs are put into cells.
The last handoff is to the test team, which uses a huge range of measurement technologies to analyze how close the programmed cell is to meeting the initial specs.
There is high variability in how well initial designs work. Some are nearly on the mark out of the gate, others require considerable improvement. The key emphasis for Ginkgo is to develop the necessary infrastructure to iterate through this Design, Build, Test loop as quickly as possible. Importantly, whether each design succeeds or fails, Ginkgo improves its data and increases its knowledge, pushing it further ahead of would-be competitors, who will need to make the same mistakes over again, and potentially speeding up future cycles.
Each turn of the crank is used to grow the Codebase and increase Foundry capacity. Here, you can see the inspiration drawn from Endy and other synthetic biology pioneers. The goal is to program cells, but the meta-goal is equally important: getting better at programming cells as quickly as possible.
From a technology perspective, the roots of many of the core ideas can be traced back to the founders’ vision for synthetic biology as a fledgling discipline, and for how to turn something like iGEM into a reality. From a business perspective, there is also a clear influence from the company’s time at YC. In 2019, Ginkgo announced a partnership with YC and Petri to offer a new deal to early-stage startups: the opportunity to launch Cell Programs with $0 down in exchange for equity.
This strategy is similar to the one that allowed Stripe to grow rapidly early on—the Collison brothers famously signed up their YC classmates by taking their computers and implementing Stripe themselves, in a move now called “the Collison installation.” Ginkgo is attempting to adapt this approach to synthetic biology R&D.
The diversity of active programs at Ginkgo gives a glimpse of what is possible with their platform today. Ginkgo and Bayer partnered to form Joyn Bio: a new company designing microbes that reduce the need for synthetic nitrogen fertilizers. This could solve a massive global bottleneck on the path towards a future with sustainable agriculture.
Beyond feeding us, synthetic biology could help us keep our bodies free of disease. Ginkgo is working with Roche to scale the discovery of antibiotics in an effort to tackle the growing problem of antibiotic resistance.
A company called Synlogic has also been an early adopter of Ginkgo’s platform. They have used the Foundry and Codebase in their efforts to create “living medicines,” which are probiotics that have been engineered to perform specific tasks in the body such as synthesizing and releasing therapeutics in response to environmental signals.
Each of these projects represents a Cell Program that is implemented with their Design Build Test engineering loop. Whether an organism is supposed to help crops metabolize nitrogen in the air, or release a drug in a specific part of the body, the starting point is a customer specification. Cell designers use the growing Ginkgo Codebase to solve this problem, stitching together genetic elements into new circuits. The circuits are instantiated in physical organisms by the build team, and tested. The major limitations are the speed at which Ginkgo can iterate on their designs, and the imagination of the customers.
So, what does The Organism Company do? Ginkgo is building a massive platform for programming cells, and making a big bet on a new business model that can make this work.
Major open questions 🐻
Ginkgo wants to become the world’s platform for programming cells. Their vision is expansive and incorporates a wide range of ideas and influences, and they don’t shy away from that. As a result, they have been met with fairly polarized responses.
For believers, Ginkgo represents a market-defining opportunity. As somebody who is focused on the importance of Sequencing, Synthesis, Scale, and Software in modern biotech, I view Ginkgo as one of the most ambitious attempts to leverage this new technology stack.
As Ginkgo has grown and gone public, they have faced a new wave of questions and skepticism about their vision and business model. After their SPAC in 2021, MIT Tech Review published a critical article questioning their valuation. Only months later, they had to weather their first attack from an activist short seller. Skeptics have only become more vocal as Ginkgo’s stock price has dropped well below its starting point over the past year.
Much of the debate about Ginkgo is centered around the fact that it is incredibly hard to accurately value their platform. There are a number of variables to consider, each with considerable uncertainty.
To start, a crucial variable to consider is the number of Cell Programs that are deployed on the platform. There are larger high-value contracts from companies like Roche and Bayer, but these partnerships can’t scale Ginkgo on their own. To generate massive profits, several of their early-stage bets on startups will have to become big successes.
In some ways, betting on Ginkgo is also betting on the vision of iGEM succeeding. For the platform to scale, they will need to help usher in an ecosystem where small teams are starting biotechs closer to the rate at which software companies are launched. The entire synthetic biology industry will need to grow and mature to much more closely resemble tech.
This leads to the next two variables that are important to consider but challenging to model: the time and cost associated with each Cell Program. In therapeutics, companies are able to recoup their massive R&D costs because of the incredibly unusual pricing aspects of drugs due to their patent exclusivity. If Ginkgo is aiming to be compensated in part by royalties or equity for products that don’t have these characteristics, they can’t afford for their R&D process to take as long or cost as much.
For these factors, enthusiasm for Ginkgo’s prospects is contingent on having confidence that they will continue to rapidly accelerate their Design Build Test loop, and that their growing Codebase will continue to simplify organism design in the future.
There are also ways that Ginkgo’s business model can even be confusing to tech investors who are more receptive to platform companies. Typically, platforms offer a service at fixed prices, and scale in revenue as their consumers scale in consumption. AWS sells computation as a service, and software companies need to continually buy more of it as they grow. Biology makes this tricky.
What may be good for the world is tougher from a business perspective. The problem is that once a customer has their designed organism, they can use it to grow as much of the programmed output as they want without Ginkgo. Imagine if you could simply grow more servers, for free, once you pay to get set up on AWS.
As Drew Endy has pointed out, “Biology is itself a way of making stuff. It's the type of material that is all over the planet, for the most part, and wherever it is, it harvests local materials and energy and makes copies of itself.” This is in part why Ginkgo’s licensing structure has to be so unique—they need a way to benefit from selling a product that grows itself.
An experiment in cooperative work structure
The ethics of running a social media platform are challenging. The ethics of running the platform for biology are even more challenging.
While Ginkgo is early in its journey, it’s already preparing for a world in which they have the great power, and great responsibility, of running that platform. To understand the scope of that responsibility, imagine all of the things that might go wrong when millions of people have easy tools to hack biology and manufacture organisms at their disposal.
Jason told us that if Ginkgo is successful and creates the platform for biology, they can’t just adopt the social media attitude that they don’t care how the platform is used. So first point: they do care ✅ What to do about it?
There are two categories of solves: technological and social.
On the technological side, Ginkgo has a responsibility to make it harder to maliciously use the platform.
They’ve done a bunch of work there, including spinning up a dedicated biosecurity unit and developed software to monitor DNA synthesis, which they compare to malware detectors in computer programming. Ginkgo caught both the BA.2 and BA.3 variants of Omicron at the airport, and were the first in the US to detect them. As synthesis becomes more widespread, Ginkgo will continue to evolve its defenses.
“Infectious diseases need to have a harder time fucking up the planet than they do today,” is how Jason succintly described the challenge.
On the social side, Ginkgo needs to make sure that the company continues to care about doing the ethical thing, even when it might pressure short-term financial performance.
Jason explained that “the people building the platform end up influencing the capabilities of the platform,” citing examples such as original heart valves being too small for women and Kodak film not capturing Black people to highlight the dangers of developing products without all of the right stakeholders involved.
So Ginkgo is staffing up to include the right people. For example, biotech has poorly served the Global South historically, so Ginkgo hires team members in Brazil. Since the company will need to fight the risk of biological weapons, it hires veterans. It hires, and plans to continue to hire, a group that represents the main stakeholders of a platform for biology.
But hiring is only one piece of the puzzle. Those employees need to be empowered to impact the direction of the platform, which is why Ginkgo introduced a model that’s rare among public companies: a co-op-like structure in which employees receive shares with 10:1 voting rights.
When Jason was in grad school at MIT, in Drew Endy’s lab, Endy gave him a paper titled Beyond Capitalism: Leland Stanford’s Forgotten Vision. The paper describes the Stanford founder’s views on the benefits of the cooperative structure, an artifact of his time in the California Gold Rush, when groups of workers formed informal co-ops to split the spoils of their discoveries. Stanford, a US Senator, introduced bills to advance cooperatives and even tried to structure the university he founded as a co-op.
The paper came back to Jason when he was working through how to address the biological platform ethics issue, and he decided to implement a modern version at Ginkgo. Here’s the logic.
In a normal corporation, shareholders have the ability to vote for board members, and board members are obligated to act in the interests of shareholders. They do that mainly by hiring and firing managers. Managers are responsible for the day-to-day operations of the business, which they largely do by exerting control over employees, who they can hire and fire. C-Corps work incredibly well for a wide range of businesses.
Instead of throwing the whole structure out and going full co-op, Jason structured Ginkgo based on this insight: if employees hold enough of a company, they can choose board members who act in the best interests of employees – the most informed shareholder base and the group with the most skin in the game. If management pushes employees to do things they believe are unethical, or simply deprioritizes things that employees believe to be important, then employees can push the board to make changes. If the board won’t make changes, the employees hold a 10x greater than average ability to change the board.
So Ginkgo gives its employees Class B shares with super-voting rights: ten votes per share versus one vote per Class A share. Companies like Meta and Google famously have similar structures, with a major difference. At those companies, executives, particularly the founders, control the majority of the votes. Zuck personally controls north of 50% of Meta’s votes. But at Ginkgo, the power extends to all currently employed team members. When an employee leaves the company, their shares convert into Class A shares. If they sell their shares, the shares convert to Class A shares.
Ginkgo isn’t trying to reinvent management, it’s just adjusting who management serves. If it hires people who are representative of the potential stakeholders of the platform for biology, and then gives those people the opportunity to impact corporate governance, it hopes to have the right checks and balances in place by the time the company will have to make very difficult decisions. It’s an example for other companies operating in the space.
And if it succeeds, it will. Because a world in which Ginkgo succeeds is going to be wild.
What will the world look like if Ginkgo succeeds?
When Jason presented Ginkgo at Y Combinator’s Demo Day in the summer of 2014, he picked his wardrobe carefully. He wore a Jurassic Park shirt.
The power to manufacture organisms that manufacture other organisms can lead in so many directions, including, conceivably, to a real-life Jurassic Park. But while it’s fun to dream about dinosaurs, Ginkgo’s success-case impact is more likely to infiltrate our everyday lives in more profound ways than prehistoric amusement.
I enjoy reading Not Boring deep dives because Packy always asks, 'What changes if this dream becomes reality?' For Ginkgo, it's hard to think about what wouldn't change if they succeed. That’s because Ginkgo isn’t just making organisms, it’s building towards a world in which anyone can program cells as easily as they program computers. “Kids today grow up interacting with electronics and learning how to program,” Ginkgo co-founder Austin Che said, “but what’s more natural for a kid, interacting with the construct of bits we’ve invented or the physical world?”
The active Cell Programs in Ginkgo’s pipeline are working to solve immediate and practical problems using microbial engineering. More sustainable agriculture, more antibiotics, smarter medicines. All of these applications are super important—but the vision of synthetic biology doesn’t stop there.
The founders and employees at Ginkgo fundamentally want to help build a new economy: the Bioeconomy. At the core, I don’t think that this is a marketing slogan, or a buzzword. It is a philosophical goal. These scientists have spent their entire careers working to establish the technology necessary for us to use the awesome power of biology to create an abundant future.
At syntheticbiology.com—a website hosted by Ginkgo—we return to the question that we started with. What if you could grow anything? This is a huge question.
For Tom Knight, the possibilities are limitless:
Important things are going to happen. Synthetic biology could be increasingly important in environmental clean-up strategies. It could also have a place in planet colonization. If it happens, then planet colonization will be reliant on synthetic biology. The words ‘insurmountable opportunities’ come to mind. Asking that question is like asking Bardeen in 1948 after developing the transistor to ‘please predict iPhones’. You can’t predict it. You can make guesses about what's important today but we don’t have a clue for the future.
Synthetic biologists are exploring ways to grow cement, home materials, or even one day grow entire homes. What if we grew organisms that ate the plastic in our oceans? What if we grew our medicines in our gardens? These types of dreams and questions represent my favorite part of synthetic biology: the unabashed and unapologetic optimism for the future.
In the Mars Trilogy by Kim Stanley Robinson, a team of scientists and engineers colonize Mars. A strong philosophical divide emerges between groups of scientists: the areologists who study Mars want to keep the planet red and untouched, while the engineers and biosphere designers want to make their new home green and full of life.
The scientists in favor of terraforming Mars cherish viriditas, which is “the green force of life, expanding into the Universe.” To them, this is the core of beauty and meaning.
Look at the pattern this seashell makes. The dappled whorl, curving inward to infinity. That's the shape of the universe itself. There's a constant pressure, pushing toward pattern. A tendency in matter to evolve into ever more complex forms. It's a kind of pattern gravity, a holy greening power we call viriditas, and it is the driving force in the cosmos. Life, you see.
Synthetic biology is a technological discipline. It is also a philosophical and aesthetic preference towards green. In a world where people are asking themselves whether it is worth having children given their eventual carbon footprint, biotechnologists are thinking of ways to mitigate climate change and grow more life in the universe instead of less. Instead of moving increasingly inwards into the world of bits, partnering with biology offers the potential to realize abundance in the world of atoms.
Thanks to Elliot for joining the team and coming out of the gate hot, and to Dan for editing and Simon Barnett for reading an early draft!
Alt Mint
Freebie time! Not Boring portfolio company Alt is launching Alt Mint. One June 17th, they’re providing early access to VIPs to mint NFTs backed by physical trading cards. Holders have the right to redeem their NFTs for trading cards ranging from $700 - $10,000 in value or to hold on to them for access to community benefits and rewards. Leore, Meredith, and the Alt team have generously offered to give 30 of those VIP spots to Not Boring readers. To enter, just fill out this form and they’ll be in touch:
Thanks for reading, and see you next week,
Packy
Recommend
About Joyk
Aggregate valuable and interesting links.
Joyk means Joy of geeK