This is the first X-ray taken of a single atom
source link: https://arstechnica.com/science/2023/05/this-is-the-first-x-ray-taken-of-a-single-atom/
Go to the source link to view the article. You can view the picture content, updated content and better typesetting reading experience. If the link is broken, please click the button below to view the snapshot at that time.
X-rays mark the spot —
This is the first X-ray taken of a single atom
SX-STM enables detection of atom type, simultaneous measurement of its chemical state.
Jennifer Ouellette - 5/31/2023, 8:07 PM
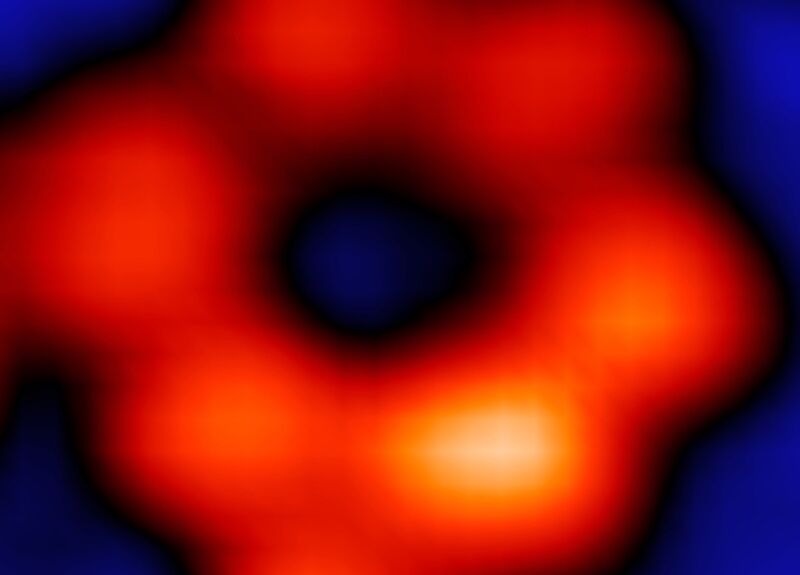
Atomic-scale imaging emerged in the mid-1950s and has been advancing rapidly ever since—so much so, that back in 2008, physicists successfully used an electron microscope to image a single hydrogen atom. Five years later, scientists were able to peer inside a hydrogen atom using a "quantum microscope," resulting in the first direct observation of electron orbitals. And now we have the first X-ray taken of a single atom, courtesy of scientists from Ohio University, Argonne National Laboratory, and the University of Illinois-Chicago, according to a new paper published in the journal Nature.
“Atoms can be routinely imaged with scanning probe microscopes, but without X-rays one cannot tell what they are made of," said co-author Saw-Wai Hla, a physicist at Ohio University and Argonne National Laboratory. "We can now detect exactly the type of a particular atom, one atom at a time, and can simultaneously measure its chemical state. Once we are able to do that, we can trace the materials down to [the] ultimate limit of just one atom. This will have a great impact on environmental and medical sciences.”
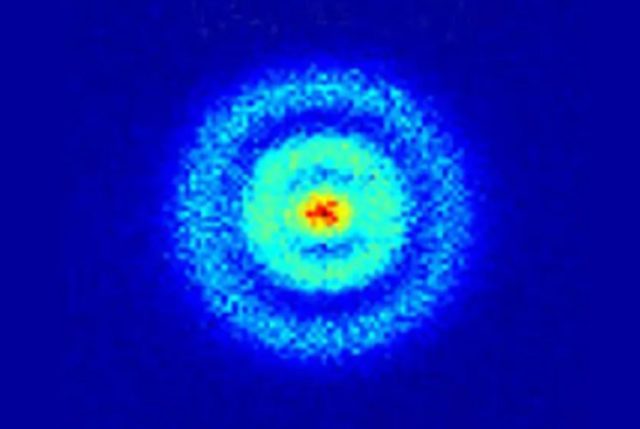
When the average non-scientist thinks of an atom, chances are they envision some popularized version of the classic, much-maligned Bohr model of the atom. That's the one where electrons move about the atomic nucleus in circular orbits, like planets orbiting the Sun in our Solar System. The orbits have set discrete energies, and those energies are related to an orbit’s size: The lowest energy, or “ground state,” is associated with the smallest orbit. Whenever an electron changes speed or direction (according to the Bohr model), it emits radiation in the specific frequencies associated with particular orbitals.
AdvertisementThe model has been superseded since Niels Bohr first proposed it in 1913, as our understanding of the quantum world advanced. Erwin Schroedinger proposed a new atomic model that dispensed with orbits in favor of energy levels. It still shares some similar concepts with the Bohr model. For instance, if an atom heats up (i.e., is energized), its electrons move to higher levels. As they cool and fall back to their normal ground state, the excess energy has to go somewhere, so it’s emitted as photons. And those photons possess frequencies that match the change in energy levels.
Technically, the electrons don’t really “move” around the nucleus in orbits. Electrons are really waves—they show up as particles when you perform an experiment to determine position—and those waves are stationary. You can check to see where an electron is, but each time you do, it will show up in a different position, not because it’s moving but because of the superposition of states. The electron doesn’t have a fixed position until you look at it, and the wave function collapses. That said, if you make a lot of individual measurements and plot the positions of the electron for each one, eventually you’ll get a ghostly orbit-like cloud pattern that is much closer to what an individual atom "looks" like.
Page:
Recommend
About Joyk
Aggregate valuable and interesting links.
Joyk means Joy of geeK