Chronic stress can inflame the gut — now scientists know why
source link: https://www.nature.com/articles/d41586-023-01700-y
Go to the source link to view the article. You can view the picture content, updated content and better typesetting reading experience. If the link is broken, please click the button below to view the snapshot at that time.

Chronic stress can inflame the gut — now scientists know why
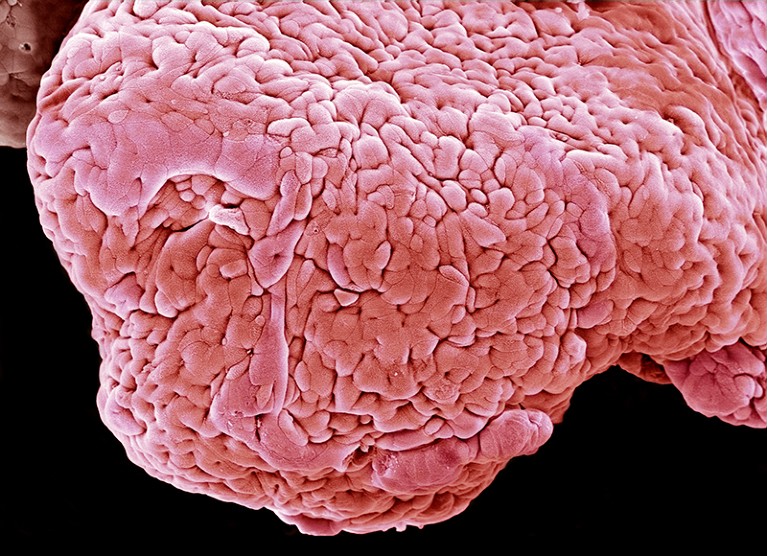
Intestinal tissue (artificially coloured) of a person with ulcerative colitis.Credit: Steve Gschmeissner/Science Photo Library
Psychological stress is known to worsen the gut inflammation caused by certain bowel diseases. Now scientists have found out why. New research1 outlines a sweeping narrative that begins with chemical cues produced in the brain and ends with immune cells in the gut — a sequence that spells trouble for people with these conditions.
The work, published today in Cell, helps to explain how chronic stress can trigger physical distress. And it implies that managing stress levels might have a profound influence over the effectiveness of treatments for inflammatory bowel disease (IBD). That idea runs contrary to conventional medical treatment, which has “completely neglected the psychological state of a patient as a major driver of [the] response to treatment”, says study co-author Christoph Thaiss, a microbiologist at the University of Pennsylvania in Philadelphia.
The path from brain to gut
Abdominal pain, diarrhoea and fatigue are just a few of the symptoms that people with IBD experience. The two main types of IBD, ulcerative colitis and Crohn’s disease, are mild in some people but, in others, can be debilitating or even life-threatening.
Stressful events, such as losing one’s job or breaking up with a partner, often precede IBD flare-ups. Thaiss and his colleagues have now traced that linkage. After a surge of stress, the brain sends signals to the adrenal glands, which release chemicals called glucocorticoids to the rest of the body.
Anxiety can be created by the body, mouse heart study suggests
Initially, the researchers considered the idea that glucocorticoids act directly on immune cells, which respond by releasing molecules that cause inflammation. “But it turns out that there is a sort of layer in between,” Thaiss says. Working in mice, they found that glucocorticoids act instead on neurons in the gut and on cells called glia that connect gut neurons to one another.
Co-opted immune cells
After being switched on by glucocorticoids, some glial cells release molecules that trigger immune cells. In turn, those immune cells release molecules that would normally be used to fight off pathogens, but in this case end up causing painful bowel inflammation.
At the same time, glucocorticoids block immature gut neurons from developing fully, the researchers found. As a consequence, these neurons produce only low levels of signalling molecules that cause gut muscles to contract. This means food moves slowly through the digestive system, which adds to the discomfort of IBD.
The researchers were surprised to learn that glucocorticoids cause gut inflammation, because these compounds are sometimes used to treat IBD. This apparent paradox might be explained by the short time frame on which such treatments are used. Although quick bursts of glucocorticoids seem to be anti-inflammatory, when stress becomes chronic, “the system completely shifts” and glucocorticoids take on a pro-inflammatory role, Thaiss says. It’s a “plausible explanation”, says gastroenterologist and immunologist John Chang at the University of California, San Diego.
Stress management for symptom relief
The brain’s ability to drive inflammation in far-flung organs “seems to be much stronger” than was thought before, Thaiss says. This suggests that IBD drugs, in combination with stress-management techniques, could be more effective than the drugs alone. Molecules in the signalling pathway that runs from the brain to the gut could also become targets for new pharmacological treatments — “an exciting possibility”, Chang says.
The implications of the work could reach beyond IBD. Stress is also thought to heighten inflammatory diseases of the skin and lungs, possibly through similar signalling pathways.
Moving forwards, Thaiss is excited to explore whether brain states other than stress influence a person’s overall health. “There’s definitely a huge amount we still need to learn about the brain and how the brain controls seemingly unrelated aspects of physiology and disease.”
Recommend
-
 12
12
Apple reportedly drags its feet when dealing with chronic China labor law offendersA new report sheds some light on how Apple deals with companies that slack on enforcing labor laws in China, alleging that if corrective actions will impact Ap...
-
 11
11
Altered Consciousness Associated with Chronic Liver Disease A 58-year-old woman came to clinic suffering from a headache, which started suddenly, and also had right hemiparesis. The patient felt drowsy while being admitted. She had no history...
-
 5
5
I’ve often found myself identifying ideas I might want to develop or pursue but yet not ready to turn that into a concrete activity. It’s a possible future avenue, but one I may (or may not) come back to in time. I want a way of taking notes...
-
 5
5
News » News & Analysis » Hypnosis app medtech raises $6.5 million to tackle menopause, depression and...
-
 17
17
A cure for Cron's chronic email problemCronicA cure for Cron's chronic email problem By Chuck Houpt The Disease: 0...
-
 16
16
Munchausen by Internet: Are chronic illness influencers really faking it?Munchausen by Internet: Are chronic illness influencers really faking it?With 15m people i...
-
 11
11
The Challenge of ‘Chronic Lyme’ Rachel Pearson When it comes to tick-borne...
-
 7
7
-
 4
4
Can apps manage our chronic health conditions?By Maddy SavageBusiness reporter, StockholmPublished4 days agoimage source, Ewa-Lena Rasmussonimage captionEwa-Lena Rasmus...
-
 1
1
How Apps Can Help People Manage Chronic IllnessesConditions such as Hashimoto’s disease often require ongoing treatment and lifestyle modifications. But apps can empower patients to improve their health.
About Joyk
Aggregate valuable and interesting links.
Joyk means Joy of geeK